Pipeline
Our initial program, HIL-214, is a virus-like particle (VLP) based vaccine candidate in development for the prevention of moderate-to-severe acute gastroenteritis caused by norovirus infection.
Norovirus
Norovirus is the most common cause of viral acute gastroenteritis (AGE) worldwide2 and is characterized by symptoms, including diarrhea, vomiting, abdominal pain, nausea, and fever, that may lead to clinically significant dehydration. The global cost of norovirus-caused AGE is estimated to be over $4 billion in direct health system costs and approximately $60 billion in societal costs per year3. In the United States alone, norovirus-caused AGE is estimated to result in $2 billion in direct medical costs and $10 billion in societal costs per year4.
Preventing the spread of norovirus is challenging as the virus can persist on environment surfaces for weeks and is resistant to common disinfectants5. While norovirus can cause illness in any age group, the majority of deaths and illnesses due to norovirus are borne by young children and older adults3. In children younger than four years of age, norovirus is estimated to cause 95,000 deaths and 450 million illnesses globally each year3. Up to 80% of children will experience a norovirus infection within one year of birth, with the majority of cases occurring between six months and two years of age6,7. For adults older than 55 years of age, norovirus is estimated to cause 78,000 deaths and 81 million illnesses globally each year3.
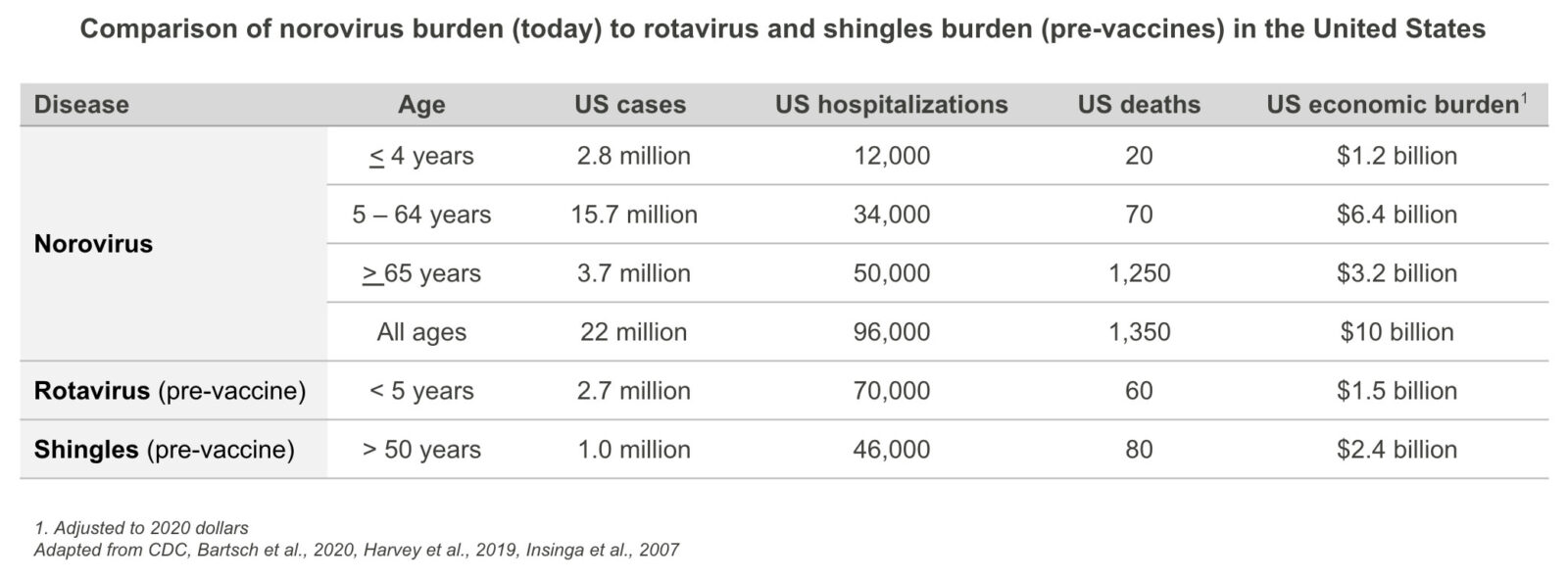
The impact of norovirus in the United States results in an average of 22 million cases of AGE, 96,000 hospitalizations, and 1,350 deaths annually4. For comparison, norovirus has a similar morbidity, mortality, and economic burden in children in the United States as rotavirus did before the introduction of rotavirus vaccines4,8. Further, norovirus today has a greater morbidity, mortality, and economic burden in United States than shingles before the introduction of shingles vaccines9,10.
HIL-214
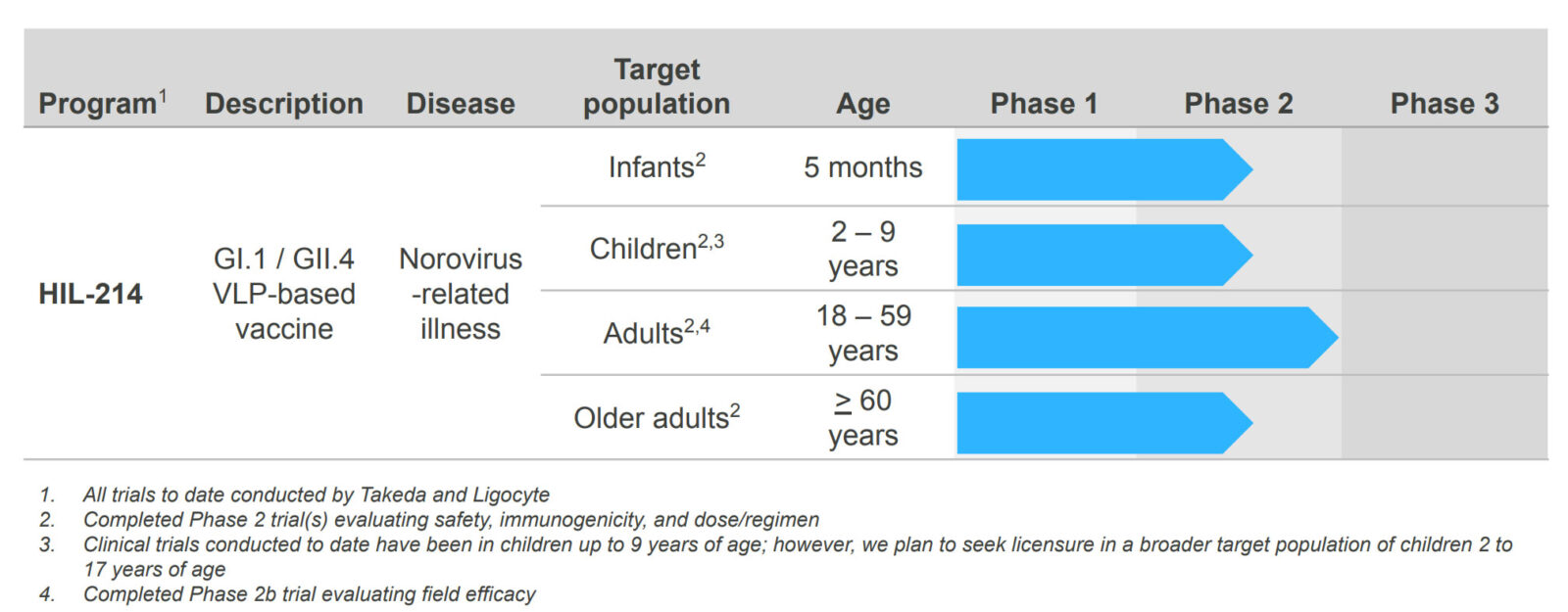
HilleVax is developing HIL-214, a norovirus bivalent GI.1/GII.4 virus-like particle (VLP) vaccine intended for active immunization for the prevention of acute gastroenteritis (AGE) caused by norovirus. The following chart summarizes our current development program:
HIL-214 has been the subject of nine Phase 1 and Phase 2 clinical trials, including more than 4,500 subjects of which more than 2,200 subjects have been evaluated for immunogenicity. These subjects have ranged from 6 weeks to 102 years old. In a randomized, placebo-controlled Phase IIb field efficacy study in 4,712 adult subjects, HIL-214 was shown to be well-tolerated and demonstrated clinical proof of concept in preventing moderate-to-severe cases of acute gastroenteritis from norovirus infection11.
KEY PUBLICATIONS
- Efficacy of an intramuscular bivalent norovirus GI.1/GII.4 virus-like particle vaccine candidate in healthy US adults
- Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults
- A phase 2 study of the bivalent VLP norovirus vaccine candidate in older adults; impact of MPL adjuvant or a second dose
- Norovirus Vaccine against Experimental Human Norwalk Virus Illness
REFERENCES
[1] Karst, S. M. Pathogenesis of Noroviruses, Emerging RNA Viruses. Viruses 2, 748–781 (2010)
[2] Centers for Disease Control (CDC). Norovirus Worldwide [Accessed on: 22 December 2021], available from: https://www.cdc.gov/norovirus/trends-outbreaks/worldwide.html
[3] Bartsch, S. M., Lopman, B. A., Ozawa, S., Hall, A. J. & Lee, B. Y. Global Economic Burden of Norovirus Gastroenteritis. Plos One 11, e0151219 (2016)
[4] Bartsch, S. M., O’Shea, K. J. & Lee, B. Y. The Clinical and Economic Burden of Norovirus Gastroenteritis in the United States. J Infect Dis 222, 1910–1919 (2020)
[5] Centers for Disease Control (CDC). CDC Vitalsigns [Accessed on: 22 December 2021], available from: https://www.cdc.gov/vitalsigns/pdf/2014-06-vitalsigns.pdf
[6] Saito, M. et al. Multiple Norovirus Infections in a Birth Cohort in a Peruvian Periurban Community. Clin Infect Dis 58, 483–491 (2014)
[7] Shioda, K., Kambhampati, A., Hall, A. J. & Lopman, B. A. Global age distribution of pediatric norovirus cases. Vaccine 33, 4065–4068 (2015)
[8] Centers for Disease Control (CDC). Rotavirus Pinkbook [Accessed on 22 December 2021], available from: https://www.cdc.gov/vaccines/pubs/pinkbook/rota.html
[9] Harvey, M., Prosser, L. A., Rose, A. M., Ortega-Sanchez, I. R. & Harpaz, R. Aggregate health and economic burden of herpes zoster in the United States: illustrative example of a pain condition. Pain 161, 361–368 (2019)
[10] Legami, V. D. et al. Epidemiology and costs of herpes zoster: Background data to estimate the impact of vaccination. Vaccine 25, 7598–7604 (2007)
[11] Sherwood, J. et al. Efficacy of an intramuscular bivalent norovirus GI.1/GII.4 virus-like particle vaccine candidate in healthy US adults. Vaccine 38, 6442–6449 (2020)